Request for Shark Tissue Samples
I have been working on a long-term collaborative project with Mahmood Shivji, of Nova Southeastern University, concerning the molecular phylogeny of sharks. We have good genetic representation of the more common carcharhinids and sphyrnids of the western North Atlantic and from Western Australia (the latter thanks to Rory McCauly). To continue and extend this work, we request shark tissue samples from as many shark species and geographic regions as possible.
Although we are grateful to receive samples from any shark species, presently, we are particularly interested in obtaining samples from the following carcharhinids and sphyrnids:
| Carcharhinus altimus | Bignose Shark |
| Carcharhinus amblyrhynchoides | Graceful Shark |
| Carcharhinus borneensis | Borneo Shark |
| Carcharhinus brachyurus | Bronze Whaler |
| Carcharhinus cautus | Nervous Shark |
| Carcharhinus dussumieri | Whitecheek Shark |
| Carcharhinus fitzroyensis | Creek Whaler |
| Carcharhinus galapagensis | Galapagos Shark |
| Carcharhinus hemiodon | Pondicherry Shark |
| Carcharhinus isodon | Finetooth Shark |
| Carcharhinus leiodon | Smooth Tooth Blacktip Shark |
| Carcharhinus macloti | Hardnose Shark |
| Carcharhinus melanopterus | Blackfin Reef Shark |
| Carcharhinus porosus | Smalltail Shark |
| Carcharhinus sealei | Blackspot Shark |
| Carcharhinus signatus | Night Shark |
| Carcharhinus sorrah | Spot-Tail Shark |
| Carcharhinus tilstoni | Australian Blacktip Shark |
| Glyphis gangeticus | Ganges Shark |
| Glyphis glyphis | Speartooth Shark |
| Glyphis siamensis | Irrawaddy River Shark |
| Isogomphodon oxyrhynchus | Daggernose Shark |
| Lamiopsis temmincki | Broadfin Shark |
| Loxodon macrorhinus | Sliteye Shark |
| Nasolamia velox | Whitenose Shark |
| Rhizoprionodon acutus | Milk Shark |
| Rhizoprionodon lalandei | Brazilian Sharpnose Shark |
| Rhizoprionodon longurio | Pacific Sharpnose Shark |
| Rhizoprionodon oligolinx | Grey Sharpnose Shark |
| Rhizoprionodon taylori | Australian Sharpnose Shark |
| Scoliodon laticaudus | Spadenose Shark |
| Triaenodon obesus | Whitetip Reef Shark |
| Eusphyra blochii | Winghead Shark |
| Sphyrna corona | Mallethead Shark |
| Sphyrna media | Scoophead Shark |
| Sphyrna tudes | Golden Hammerhead |
Protocol for Elasmobranch Tissue
Collection for Genetic Studies
The basic protocol is very straight forward. There are two very important steps to keep in mind:
1. The species identity and other information (sex, length if available, and approximate geographic location of capture) must be recorded correctly. If the identity of the species is uncertain, please note that on the sample tube and on the data sheet accompanying the samples.
2. It is absolutely critical that cross-contamination of tissues from one animal to another does not occur. The best way to ensure that cross-contamination does not occur is to use a new cutting tool (e.g. scalpel or razor blade) for each animal. The other option is to rinse and clean off the cutting tool (e.g. knife or scissors) between sampling each animal.
Sampling Steps:
1. The best tissue sample is 1 or 2 pieces of fin (dorsal, pectoral, or pelvic) about the size of a thumbnail. A fin clip can be taken without sacrificing the animal. If the animal is to be sacrificed for other studies, then heart and muscle (in that order) are also good tissues for DNA. Fin is best.
2. Place sampled tissue in vial provided and record species and sex - plus other data (capture location, length if available) — in pencil on a piece of paper and slip that in the vial, too. Do not over-pack the vials with tissues: the preservative (either 95% reagent grade ethanol or a solution of EDTA, DMSO, NaCl) should be able to flow around the sample. If the sample is large, please cut it into smaller pieces to fit the vials.
3. Please make sure the cap on each vial is closed tightly to prevent leaks during transportation.
4. Vials with sample can be kept at room temperature (out of direct sunlight).
Supporting Photography:
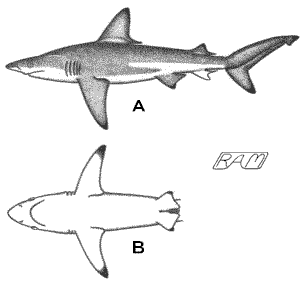 If in doubt, however slightly, of a species
identification, place a numbered or lettered card next to the specimen and
photograph them together. Two views of each specimen are ideal: A) complete lateral (side) view, and B) underside of head and
pectoral fins. Write the corresponding number or letter onto the specimen
data sheet and insert it into the vial with the tissue sample from that
animal. This will facilitate identification of the source species later.
If in doubt, however slightly, of a species
identification, place a numbered or lettered card next to the specimen and
photograph them together. Two views of each specimen are ideal: A) complete lateral (side) view, and B) underside of head and
pectoral fins. Write the corresponding number or letter onto the specimen
data sheet and insert it into the vial with the tissue sample from that
animal. This will facilitate identification of the source species later.
Submission:
Send tissue samples and photographs to:
R. Aidan Martin
ReefQuest Centre for Shark Research
P.O. Box 48561
595 Burrard Street
Vancouver, BC V7X 1A3
C A N A D A
